What is Endocytosis?
Endocytosis is an essential process serving multiple key cellular functions, such as nutrient uptake, signal transduction, and defense against pathogens. In endocytosis, a plasma membrane patch is internalized, forming a transport vesicle containing cargo from the extracellular space. Transport vesicles bud off from the plasma membrane and eventually fuse with a target cellular compartment. Transport vesicles must be highly selective in recognizing the correct target membrane for fusion to ensure correct cargo delivery. Therefore, the vesicles harbor specific surface markers identifying their origin and type of cargo, whereas target membranes display the corresponding receptors. In this process, Rab proteins direct the vesicle to the specific spot on the target membrane, where tethering proteins recognize the vesicles and pull cargo and targeting membrane close together. Finally, SNARE proteins mediate membrane fusion.

The cell internalizes cargo molecules by invaginating the plasma membrane, forming vesicles. Vesicles can follow the pathway all the way to lysosomes for degradation, or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans-Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.
The role of Rab proteins in organelle biogenesis and transport
Rab proteins are the largest subfamily of small GTPases. Each Rab protein is associated with one or more membrane-enclosed organelles in the secretory or endocytic pathway. Rab GTPases are regulated by GTPase-activating proteins (GAPs) and guanine exchange factors (GEFs) which bind to Rab and cause GDP release. Rab proteins cycle between the cytosol in their GDP-bound inactive state and a membrane in their GTP-bound active state. Membrane-bound Rab-GEFs activate Rab on target membranes, where they then assemble protein complexes. The Rab binding partners on the membrane are called Rab effectors and facilitate vesicle transport, membrane tethering, and fusion. The assembly of a Rab protein together with its effectors on their target membrane forms a Rab domain and occupies a distinct membrane subdomain on the organelle.
The components of the Rab5 machinery
Over the last decades, our research group has focussed on deciphering the interactome and mechanism by which one of the Rab proteins, Rab 5, provides membrane specificity and facilitates targeting and membrane fusion with early endosomes.
Important insight into the interactome of Rab5 came from the biochemical purification of a large set of Rab5 interacting proteins (Christoforidis et al., 1999). We found that Rab5 regulates a molecular network of over 40 different effector and regulator proteins. Each of these proteins has a specific function in vesicle formation, tethering, fusion, or cytoskeleton-dependent motility of early endosomes. Subsequent work showed that Rab5 effectors function cooperatively, thus the activity of one effector is essential for the activity of another. For example, the Phosphoinositide 3-kinase (PI3K) Vps34 is required for the synthesis of phosphatidylinositol (3)-phosphate (PI(3)P) in the membrane. Afterward, PI(3)P recruits FYVE domain-containing proteins, such as EEA1, to bind to the membrane of early endosomes interacting with PI(3)P.
Rab proteins organize in distinct domains on membranes
Each member of the Rab protein family occupies a distinct membrane domain in the secretory and endocytic pathways. Rab4, Rab5, and Rab11 form distinct domains on early endosomes (Sönnichsen et al., 2000) and therefore organize the endosomal system in a modular roadway – each Rab domain fulfilling a specific set of functions. A binary switch system employing divalent Rab effectors, such as Rabaptin-5 (Vitale et al., 1998) and Rabenosyn-5 (De Renzis et al., 2002) connects one Rab GTPase to another, allowing the sequential transport of cargo between adjacent Rab domains (Zerial and McBride, 2001).
Rab effectors cluster in Rab membrane domains
The assembly of a Rab5 domain on a target membrane involves a complex cascade of molecular interactions. Recent advances in microscopy techniques allowed us to map Rab5c and markers for the recycling and degrading cargo routes to the ultrastructure of an early endosome with nanoscale resolution using correlative single-molecule localization microscopy (SMLM) electron microscopy (superCLEM) (Franke et al., 2019). This data suggests that early endosomes have multiple Rab5c domains that vary in number and size. We have now succeeded in reconstituting such domains in vitro with a minimal machinery including Rab5, Rab GDI, and the Rabaptin5-Rabex5 complex.
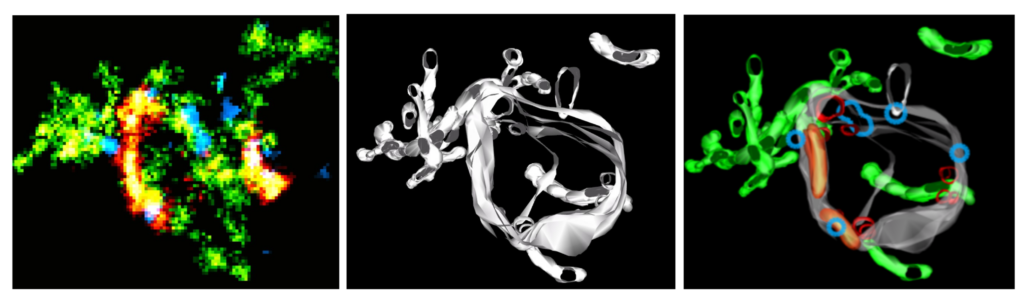
These images show a superresolution light microscopy signal (left) and an ultrastructural model of an early endosome based on electron tomographs (middle). On the right, you can see an overlay of both images mapping the light microscopy signals to the structure. You can see the delimiting endosomal membrane (grey), intraluminal vesicles (red), endosomal recycling tubules (green), sorting microdomains (orange), and Rab5c domains (blue). Adapted after Franke et al., 2019.
Unexpected dynamics of Rab domains: Rab5-Rab7 conversion
Rab5 and Rab7 are key determinants of early and late endosomes, organizing effector proteins into specific membrane subdomains. Once assembled, is the Rab machinery indefinitely maintained, or does disassemble in the course of cargo transport? In 2005, we found the level of Rab5 dynamically fluctuates on individual early endosomes, linked by fusion and fission events into a network in time. Progression of cargo from early to late endosomes occurs via the replacement of Rab5 with Rab7, a process termed Rab conversion. These findings led us to propose a model whereby organelle transport requires the sequential flow of assembly and disassembly of Rab GTPases and their effectors on the membrane (Rink et al., 2005).
An in vitro reconstitution system to study the role of Rab5 in membrane fusion
The canonical model of membrane fusion suggests that SNAREs are sufficient to dock and fuse membranes in vitro. However, Rab GTPases and their effectors are essential components acting at an earlier stage, such as tethering between membranes and priming and pairing of SNAREs. We developed an in vitro vesicle fusion assay including Rab5, its key regulators and effectors, and early endosomal SNAREs. These vesicles could fuse with purified early endosomes or with each other, but membrane fusion with SNAREs alone was almost undetectable and required cooperativity between Rab5 effectors and SNAREs, implying that the Rab5 machinery is a core component of membrane fusion and endosome biogenesis (Ohya et al., 2009). More recently, we used the reconstitution system to study the self-organization of Rab5 and its GEF/effector complex Rabex5/Rabaptin5 on supported bilayers (Cezanne et al., 2020). We discovered that both protein and lipid interactions are necessary for patterning.
The membrane tether EEA1 generates an entropic collapse force
Recently, we have been working on the mechanism by which the tethering protein EEA1 mediates membrane fusion. We found that Rab5 binding to EEA1 induces an allosteric conformational change, generating an entropic collapse force that we measured with the help of optical tweezers (Murray et al., 2016). This force might help pull the membranes together before they finally fuse. We now show that EEA1 can be recycled to its extended conformation without the input of chaperones, suggesting that Rab5 and EEA1 constitute a coupled two-component molecular motor that does mechano-chemical work (Sing and Soler et al., 2023).

Localization of the FERRY complex (yellow/ red) in neurons. Adapted from Schuhmacher et al., 2023.
The FERRY protein complex links mRNA to early endosomes
Cells are responsible for producing proteins throughout their structures, but the process becomes particularly complex in long cells like axons of neurons. Recently, we discovered a new protein complex called FERRY. In neurons, FERRY is associated with early endosomes and functions similarly to a tie-down strap during transport. It directly interacts with mRNA and keeps it attached to the early endosomes, which then act as carriers for mRNA distribution (Schuhmacher et al., 2023). The question arises: how does FERRY actually bind to mRNA? To answer this, a collaborator employed cryo-electron microscopy to examine the structure of FERRY and identify the molecular characteristics that enable the complex to bind to both early endosomes and mRNAs (Quentin et al., 2023).
Rab5 is necessary for the biogenesis of the endo-lysosomal system in vivo
Our in vitro studies demonstrated that Rab5 can assemble a multi-protein machinery on the endosome membrane that, together with SNARES, mediates efficient membrane tethering and fusion. We showed that loss of Rab5 in the adult mouse liver caused a dramatic reduction in the number of early endosomes, late endosomes, and lysosomes. Additionally, mice depleted of Rab5 in the liver exhibited various metabolic phenotypes (Zeigerer et al., 2012). We are currently using biochemistry, cell, and systems biology methods to elucidate the interesting link between endocytosis and metabolism and characterize genes involved in the regulation of hepatocyte polarity.







